UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 7, 2019
AVROBIO, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-38537 | 81-0710585 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
One Kendall Square
Building 300, Suite 201
Cambridge, MA 02139
(Address of principal executive offices, including zip code)
(617) 914-8420
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading symbol(s) |
Name of each exchange on which registered | ||
| Common Stock, $0.0001 par value per share | AVRO | Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On October 7, 2019, AVROBIO, Inc. updated its corporate presentation for use in meetings with investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01 and Exhibit 99.1 attached hereto is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| 99.1 | AVROBIO, Inc. slide presentation, dated October 7, 2019. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| AVROBIO, INC. | ||||||
| Date: October 7, 2019 | By: | /s/ Geoff MacKay | ||||
| Geoff MacKay | ||||||
| President and Chief Executive Officer | ||||||

Freedom from a lifetime of disease Corporate Presentation October 2019 Exhibit 99.1

Disclaimer This presentation has been prepared by AVROBIO, Inc. (“AVROBIO”) for informational purposes only and not for any other purpose. Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and AVROBIO’s own internal estimates and research. While AVROBIO believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and AVROBIO makes no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. While AVROBIO believes its internal research is reliable, such research has not been verified by any independent source. This presentation may contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts”, “goal,” “intends,” “may” “plans,” “possible,” “potential,” “seeks,” “will,” and variations of these words or similar expressions that are intended to identify forward-looking statements. These forward-looking statements include, without limitation, statements regarding our business strategy, prospective products and goals, the therapeutic potential of our investigational gene therapies, the design, enrollment and timing of ongoing or planned clinical trials, clinical trial results, product approvals and regulatory pathways, potential regulatory approvals and the timing thereof, anticipated benefits of our gene therapy platform, the expected safety profile of our investigational gene therapies, timing and likelihood of success, plans and objectives of management for future operations, future results of anticipated products, and the market opportunity for our investigational gene therapies. Any such statements in this presentation that are not statements of historical fact may be deemed to be forward-looking statements. Any forward-looking statements in this presentation are based on AVROBIO’s current expectations, estimates and projections about our industry as well as management’s current beliefs and expectations of future events only as of today and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to, the risk that any one or more of AVROBIO’s investigational gene therapies will not be successfully developed or commercialized, the risk of cessation or delay of any ongoing or planned clinical trials of AVROBIO or our collaborators or of encountering challenges in the enrollment or dosing in such clinical trials, the risk that AVROBIO may not realize the intended benefits of our gene therapy platform, the risk that our investigational gene therapies or procedures in connection with the administration thereof will not have the safety or efficacy profile that we anticipate, the risk that prior results, such as signals of safety, activity or durability of effect, observed from preclinical or clinical trials, will not be replicated or will not continue in ongoing or future studies or trials involving AVROBIO’s investigational gene therapies, the risk that we will be unable to obtain and maintain regulatory approvals for our investigational gene therapies, the risk that the size and growth potential of the market for our investigational gene therapies will not materialize as expected, risks associated with our dependence on third-party suppliers and manufacturers, risks regarding the accuracy of our estimates of expenses and future revenue, risks relating to our capital requirements and needs for additional financing, and risks relating to our ability to obtain and maintain intellectual property protection for our investigational gene therapies. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause AVROBIO’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in AVROBIO’s most recent Quarterly Report on Form 10-Q, as well as discussions of potential risks, uncertainties and other important factors in AVROBIO’s subsequent filings with the Securities and Exchange Commission. AVROBIO explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law. Note regarding trademarks: plato is a trademark of AVROBIO. Other trademarks referenced in this presentation are the property of their respective owners.

Deep pipeline targeting lysosomal storage disorders (LSDs) where SoC ~$4B 2018 net sales Compelling Fabry data across Phase 1 and Phase 2 trials Gaucher and cystinosis trial recruitment underway Powered by plato™ - our commercial-stage manufacturing platform Management comprised of cell, gene and rare disease industry leaders Multiple near-term milestones anticipated Developing gene therapies designed to cure rare diseases

Cell, gene and rare disease industry leaders MANAGEMENT TEAM BOARD OF DIRECTORS Geoff MacKay President and CEO Kim Warren, PhD Head of Operations Chris Mason, MD, PhD, FRCS Chief Science Officer Deanna Petersen, MBA Chief Business Officer Kathryn McNaughton, PhD SVP Portfolio & Program Management Birgitte Volck, PhD, MD President of Research and Development Erik Ostrowski Chief Financial Officer Steven Avruch, JD General Counsel Josie Yang, PhD Head of Regulatory Affairs Bruce Booth, DPhil Chairman Ian Clark Philip Vickers, PhD Annalisa Jenkins, MBBS, FRCP Phillip Donenberg Chris Paige, PhD Geoff MacKay Georgette Verdin Chief Human Resources Officer

Steady stream of clinical programs Investigational Gene Therapy Proof-of-Concept IND-Enabling Phase 1/2 Commercial Rights Fabry AVR-RD-01 AVROBIO Gaucher AVR-RD-02 AVROBIO Cystinosis AVR-RD-04 AVROBIO Pompe AVR-RD-03 AVROBIO 4 clinical trials up and running Phase 1 Phase 2 Phase 1/2 Phase 1/2 Pre-Clinical
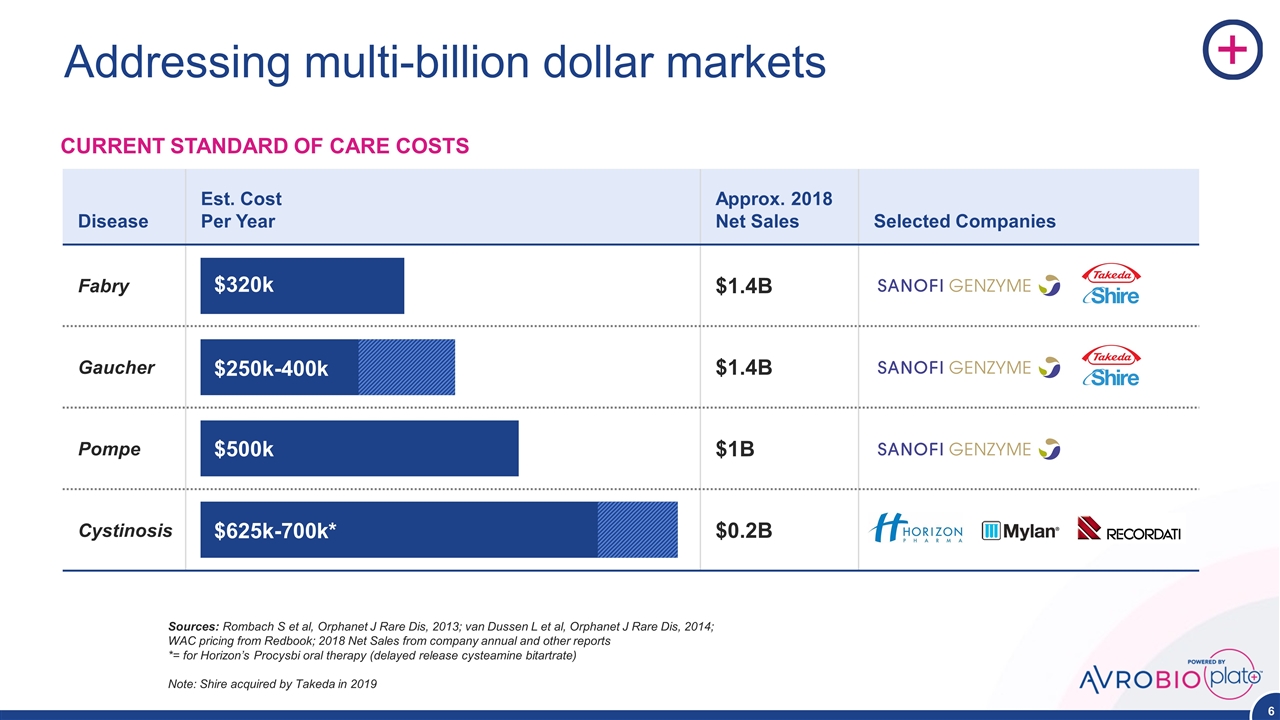
Addressing multi-billion dollar markets Disease Est. Cost Per Year Approx. 2018 Net Sales Selected Companies Fabry $1.4B Gaucher $1.4B Pompe $1B Cystinosis $0.2B CURRENT STANDARD OF CARE COSTS Sources: Rombach S et al, Orphanet J Rare Dis, 2013; van Dussen L et al, Orphanet J Rare Dis, 2014; WAC pricing from Redbook; 2018 Net Sales from company annual and other reports *= for Horizon’s Procysbi oral therapy (delayed release cysteamine bitartrate) Note: Shire acquired by Takeda in 2019 $320k $250k-400k $500k $625k-700k*

Life-long treatments vs. potential single dose cure Treatment burden Enzyme or protein level DISEASE PROGRESSION CONTINUES DISEASE PROGRESSION COULD HALT Enzyme Replacement Therapy (ERT) Temporary bolus of enzyme, not curative Bi-Weekly ERT Plasma Pharmacokinetics of ERT Life-long infusions Transient, intermittent elevation Bi-weekly IV infusions AVROBIO Gene Therapy 24/7 expression of protein, curative potential Functional Protein Expression in Transduced HSCs and Their Progeny 24/7 expression One-Time Gene Therapy Long-term, continuous elevation Single IV infusion

One platform applied across our portfolio Transduce with lentiviral vector carrying normal gene GENE THERAPY PLATFORM Select CD34+ stem cells Collect mobilized blood 3 2 1 Harvest and freeze 4 Infuse 5

Endogenous enzyme delivered to tissues via multiple cell lineages Long-term engraftment in bone marrow Manufacturing, transportation and delivery in blood Example target organ T NK B RBC Platelets Granulocytes DC Monocytes Kidney glomerulus LEUKOCYTES

Two AVR-RD-01 Fabry clinical trials 8 patients dosed across Phases 1 and 2 PHASE 1 Investigator-Sponsored Trial* PHASE 2 AVRO – FAB-201 Trial Patients Patients Key Objective Key Objectives n = 5 (fully enrolled) On ERT > 6 months prior to enrollment 18 - 50 year-old males n = 8-12 (3 patients dosed to-date) Treatment-naive 16 - 50 year-old males Safety and preliminary efficacy Safety and efficacy * Sponsored by FACTs team (Fabry Disease Clinical Research and Therapeutics) in Canada

FAB-201 Primary and secondary endpoints FAB-201 Primary efficacy endpoint Average number of Gb3 inclusions per kidney peritubular capillary (PTC) Biopsy at 1 year vs. baseline FDA-recognized endpoint in Fabry BIOMARKERS Toxic metabolite – lyso-Gb3 in plasma, urine Substrate – Gb3 in plasma, urine, skin Enzyme – AGA in leukocytes, plasma VCN PATIENT WELL-BEING Clinical status Quality of life ORGAN AND SYSTEM FUNCTION Kidney function Cardiac function GI distress Pain Secondary efficacy endpoints Gb3, also referred to as GL-3: a type of fat that builds in cells, resulting in damage to kidneys, heart and brain Peritubular capillaries (PTCs), also referred to as kidney interstitial capillaries (KICs) convey blood after filtration in the glomeruli, enabling it to eventually exit the kidneys and return to the circulatory system AEs, SAEs Clinical labs, ECG, vital signs Antibodies, RCL, ISA Primary safety endpoints

PATIENT 1 PATIENT 2 PATIENT 3 Age symptom onset / diagnosis 10 / 19 years 36 / 37 years 13 / 13 years Age dosed with AVR-RD-01 21 years 46 years 40 years Mutation c.1021G>A (p.E341K) c.644A>G (p.N215S) c.639+1G>T Primary disease signs and symptoms Kidney disease Chronic pain GI symptoms Decreased cold sensation Cardiac disease Peripheral neuropathy Chronic pain Increased tiredness GI symptoms Intermittent tinnitus Mild high frequency hearing loss Raynaud’s syndrome Kidney disease GI symptoms Peripheral neuropathy Bilateral deafness Tinnitus Peripheral edema Decreased cold sensation Leukocyte AGA enzyme activity at baseline (nmol/h/mg) 0.10* 2.38** 0.58** Plasma lyso-Gb3 at baseline (nM)*** 202 8 147 Comment IgA deposits in kidney biopsy Cardiac variant, not a classic Fabry male FAB-201 Patient Characteristics * Mayo Lab, ref range ≥23.1 nmol/h/mg ** Rupar Lab, ref range 24-56 nmol/h/mg *** Reference value ≤ 2.4 nM

FAB-201 Patient 1: 87% substrate reduction in kidney biopsy Average number of Gb3 inclusions per peritubular capillary (PTC) Baseline 1 Year (48 weeks) Baseline: The last available, non-missing observation prior to AVR-RD-01 infusion Note: With respect to Fabry disease, Gb3 inclusions per PTC is interchangeable with GL-3 inclusions per KIC FAB-201-1: First patient in FAB-201 clinical trial 3.55 0.47 Unpaired t test for difference between n=55 PTCs at baseline vs. n=101 PTCs at 1 year; p < 0.0001 Error bar represents the standard deviation 3.55

FAB-201 Patient 1: Continued reduction in substrate inclusions in skin endothelial cells Source: Thurberg BL, 2011, https://everylifefoundation.org/wp-content/uploads/images/workshopseries/16-Thurberg-Fabry-pathology-Nov-2011-compr-dc.pdf Severe accumulation Mild accumulation Moderate accumulation Biopsy Score Baseline Month 6 Month 12 Trace or no accumulation

KIDNEY FUNCTION remains within normal range FAB-201 Patient 1: Kidney and cardiac function stable at one year CARDIAC FUNCTION remains within normal range Reference Range Mean Values ± SD EF (%) LVM (g) LVMI (g/m2) Male (20-39 years) 64.3 ± 4.2 138.9 ± 24.5 67.8 ± 10.7 mGFR mL/min/1.73 m2 eGFR mL/min/1.73 m2 EF (%) LV Mass (Absolute) (g) LV Mass Index (Normalized) (g/m2) Normal Range mGFR/eGFR Male (20-29 years) Average 116* Source: https://www.kidney.org/atoz/content/gfr Note: mGFR is measured Glomerular Filtration Rate, eGFR is estimated Glomerular Filtration Rate Source: Alfakih K et al, J Magn Reson Imaging, 2003 Note: EF is Ejection Fraction, LVMI is Left Ventricular Mass Index

Gene Therapy FAB-201 Patient 1: Substantial reduction in plasma substrate / metabolite levels, sustained at 1 year Plasma Lyso-Gb3 87% Reduction Plasma Gb3 73% Reduction Gene Therapy Infuse AVR-RD-01 Day 0 Baseline: The last available, non-missing observation prior to AVR-RD-01 infusion Note: AVR-RD-01 is an investigational gene therapy nM nM Infuse AVR-RD-01 Day 0

FAB-201 Patient 1: Sustained leukocyte and plasma enzyme activity at 1 year; VCN stable Infuse AVR-RD-01 Day 0 Plasma AGA Activity Plasma AGA Activity (nmol/hr/mL) Leukocyte AGA Activity (nmoles/hr/mg) Day 0 VCN VCN (per cell) Leukocyte AGA Activity Infuse AVR-RD-01 Note: 0.1 VCN is indicative of approx. 5-10% of all nucleated cells having an average of 1-2 copies of the transgene Baseline: The last available, non-missing observation prior to AVR-RD-01 infusion
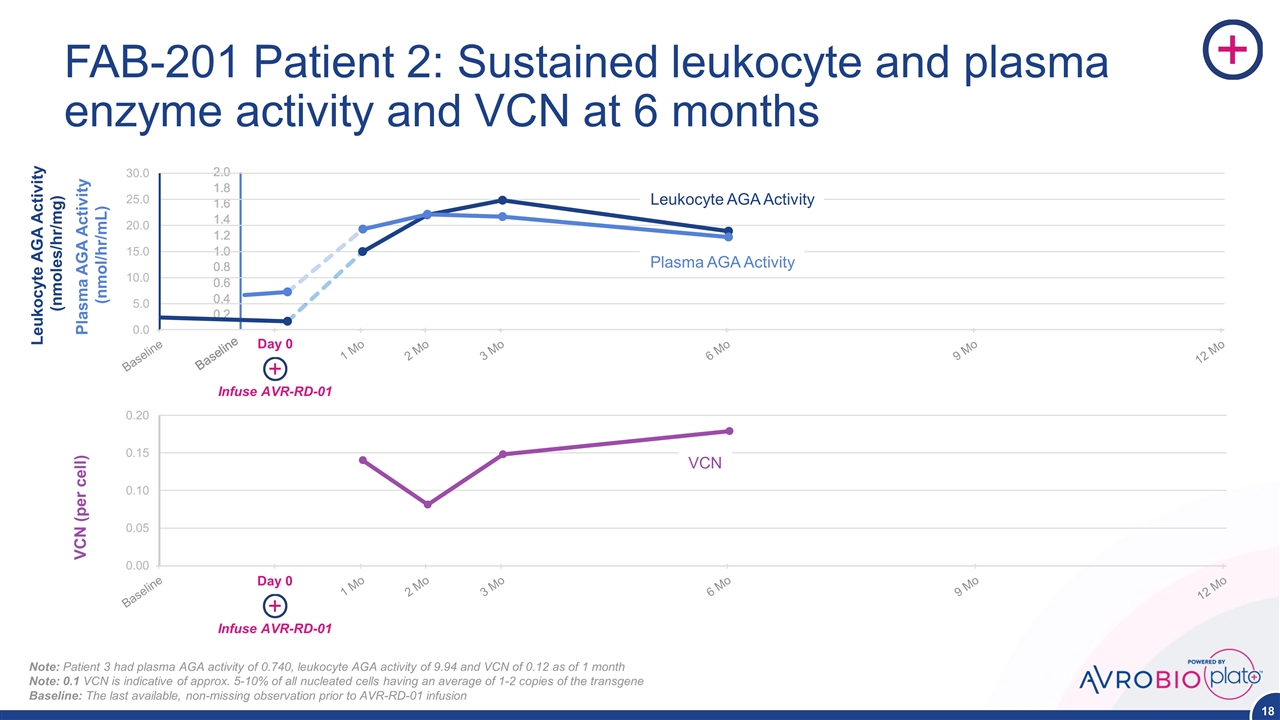
FAB-201 Patient 2: Sustained leukocyte and plasma enzyme activity and VCN at 6 months Day 0 VCN Leukocyte AGA Activity Plasma AGA Activity Infuse AVR-RD-01 Day 0 Infuse AVR-RD-01 Plasma AGA Activity (nmol/hr/mL) Leukocyte AGA Activity (nmoles/hr/mg) VCN (per cell) Note: Patient 3 had plasma AGA activity of 0.740, leukocyte AGA activity of 9.94 and VCN of 0.12 as of 1 month Note: 0.1 VCN is indicative of approx. 5-10% of all nucleated cells having an average of 1-2 copies of the transgene Baseline: The last available, non-missing observation prior to AVR-RD-01 infusion

Anti-AGA antibodies Transient low titer in 1 subject (resolved) AEs and SAEs reported AEs Generally consistent with myeloablative conditioning, underlying disease or pre-existing conditions SAEs Pre-treatment Seizure (resolved) Post-treatment Dehydration, nausea, vomiting (resolved) Febrile neutropenia (resolved) FAB-201 3 patients dosed No unexpected trends or safety events identified No AEs or SAEs related to AVR-RD-01 drug product Note: Safety database cut as of July 10, 2019

Two AVR-RD-01 Fabry clinical trials 8 patients dosed across Phases 1 and 2 PHASE 1 Investigator-Sponsored Trial* Patients Patients Key Objective Key Objectives n = 5 (fully enrolled) On ERT > 6 months prior to enrollment 18 - 50 year-old males n = 8-12 (3 patients dosed to-date) Treatment-naive 16 - 50 year-old males Safety and preliminary efficacy Safety and efficacy * Sponsored by FACTs team (Fabry Disease Clinical Research and Therapeutics) in Canada PHASE 2 AVRO – FAB-201 Trial

Phase 1 Patient Characteristics PATIENT 1 PATIENT 2 PATIENT 3 PATIENT 4 PATIENT 5 Age symptom onset / diagnosis 18 / 37 9 / 29 10 / 0 7 / 4 10 / 14 Years on ERT 11 6 4 11 2 Age dosed with AVR-RD-01 48 39 40 37 30 Mutation c.962A>G (p.Q321R) c.1033T>C (p.S345P) c.427G>C (p.A143P) c.427G>C (p.A143P) (p.Y134S) Primary disease signs and symptoms Kidney disease Cardiac disease GI pain GI diarrhea Angiokeratoma Insomnia Kidney disease Cardiomyopathy Hypohidrosis Corneal verticillata Peripheral neuropathy GI symptoms Angiokeratoma Lymphedema Acroparesthesia Cardiac Disease Tinnitus Headaches Dizziness Acroparesthesia Cardiac Disease Hypohidrosis Tinnitus Corneal verticillata Angiokeratoma GI symptoms Kidney disease Hypertension Hypohidrosis Tinnitus Migraines Impaired hearing Angiokeratoma Sleep apnea Asthma Depression Leukocyte AGA activity at baseline* (nmol/h/mg) 2.1 1.1 0.6 2.2 1.0 Plasma lyso-Gb3 at baseline (nM)** 25 26 59 29 16 Discontinued ERT 18 months after gene therapy dose Did not resume ERT after gene therapy dose 7 months after gene therapy dose * Rupar Lab, ref range 24-56 nmol/h/mg ** Reference value ≤ 2.4 nM

Phase 1: Plasma lyso-Gb3 reduction sustained >2 yrs Reduced 41% from ERT baseline* ERT No ERT Gene Rx ERT + Gene Therapy Gene Therapy Infuse AVR-RD-01 ERT Discontinued ERT-Lyso-Gb3 Range *Baseline: The mean of the values reported prior to initiating mobilization Note: AVR-RD-01 is an investigational gene therapy candidate Day 0 18 Mo Patient #1
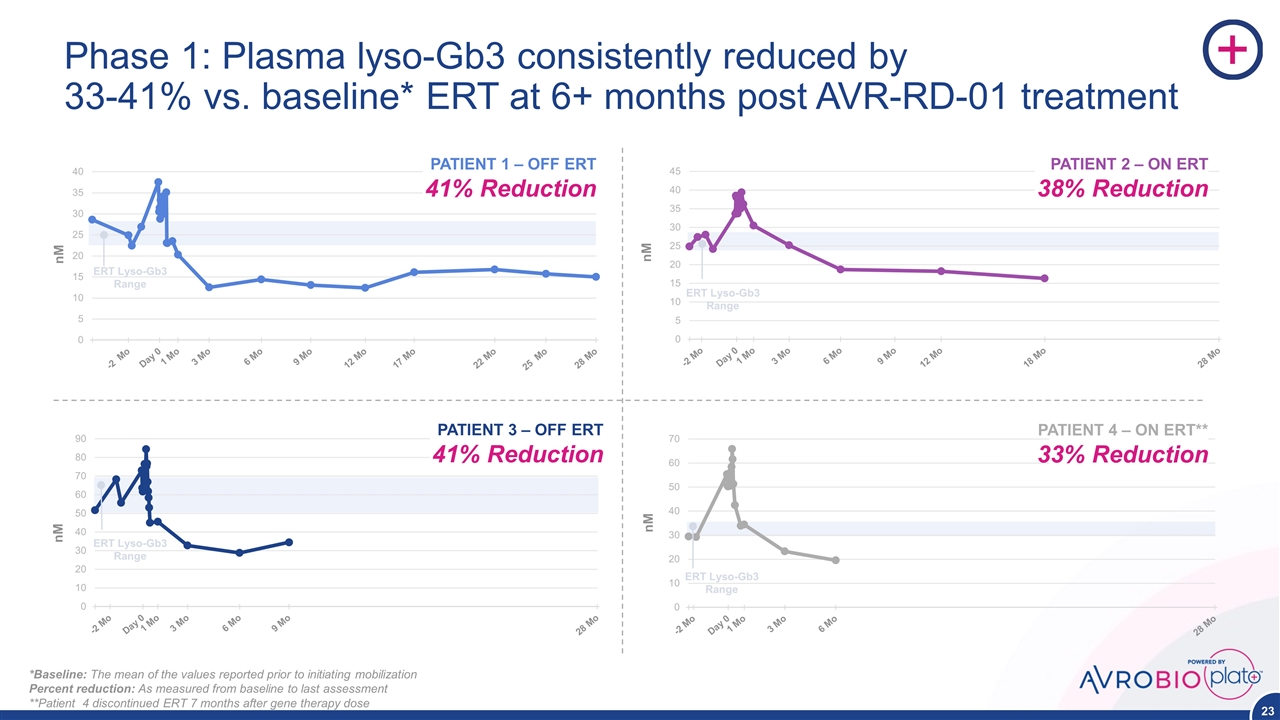
Phase 1: Plasma lyso-Gb3 consistently reduced by 33-41% vs. baseline* ERT at 6+ months post AVR-RD-01 treatment PATIENT 1 – OFF ERT 41% Reduction PATIENT 3 – OFF ERT 41% Reduction PATIENT 2 – ON ERT 38% Reduction ERT Lyso-Gb3 Range Baseline PATIENT 4 – ON ERT** 33% Reduction *Baseline: The mean of the values reported prior to initiating mobilization Percent reduction: As measured from baseline to last assessment **Patient 4 discontinued ERT 7 months after gene therapy dose ERT Lyso-Gb3 Range ERT Lyso-Gb3 Range ERT Lyso-Gb3 Range

Phase 1: Leukocyte and plasma enzyme activity sustained >2 years; VCN stable VCN Infuse AVR-RD-01 Day 0 Plasma AGA Activity (nmol/hr/mL) Leukocyte AGA Activity (nmoles/hr/mg) VCN (per cell) Plasma AGA Activity Leukocyte AGA Activity Patient #1 Infuse AVR-RD-01 Day 0 Note: 0.1 VCN is indicative of approx. 5-10% of all nucleated cells having an average of 1-2 copies of the transgene

Phase 1: Leukocyte and plasma enzyme activity levels trend consistently across all patients Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 Day 0 Infuse AVR-RD-01 Day 0 Note: Enzyme measurements are taken at ERT troughs; Note: Dotted line illustrative only Patient #5’s Day 12 data point was utilized since the one month data was not obtained 28 Mo

Phase 1: Consistent VCN trend across all patients Drug Product VCN Patient 1 0.7 Patient 2 1.4 Patient 3 0.8 Patient 4 1.4 Patient 5 1.2 Note: 0.1 VCN is indicative of approx. 5-10% of all nucleated cells having an average of 1-2 copies of the transgene Patient 1 Patient 2 Patient 3 Patient 4 Patient 5

Anti-AGA antibodies Mild titer rise in 1 patient AEs and SAEs reported AEs Generally consistent with myeloablative conditioning, underlying disease or pre-existing conditions SAEs Febrile neutropenia (resolved) Thrombophlebitis (resolved)* Phase 1 5 patients dosed No unexpected trends or safety events identified No SAEs related to AVR-RD-01 drug product Note: Safety database cut as of May 24, 2019 *Resolved post-safety database cut-off date

Emerging data support potential first-line use in Fabry disease 87% decrease in Gb3 in first kidney biopsy at 1 year in first Phase 2 patient Plasma lyso-Gb3 reduced by 30-40% vs. baseline ERT in four Phase 1 patients No unexpected trends or safety events identified 8 patients across 2 trials Kidney and cardiac function stable at 1 year in first Phase 2 patient 8 patients dosed across 2 trials longest follow-up >2 years Durability sustained >2 years for enzyme activity and VCN in first Phase 1 patient

GAU-201: Phase 1/2 study in Gaucher Type 1 patients GAUCHER DISEASE TYPE 1 ERT-STABLE and TREATMENT NAÏVE PATIENTS OBJECTIVES PATIENTS ASSESS Safety Engraftment Efficacy (functional endpoints and biomarkers) Evaluate need for ERT re-initiation 8-16 patients 16-35 year old males and females Two arms Treatment naïve Stable receiving ERT Vector Copy Number (VCN) Chimerism GCase activity, including in CSF Efficacy Hematologic values End-organ volumes and BMD Biomarkers and QoL Safety An adaptive, open-label, multinational phase 1/2 study of the safety and efficacy of ex vivo, lentiviral vector mediated gene therapy AVR-RD-02 for patients with Type 1 Gaucher disease Day -4 Conditioning Day 0 Infuse AVR-RD-02 Day 28 Post-treatment assessment Months 3, 6, 9 & 12 Safety & efficacy assessments Day -60 Mobilize stem cells Patients on ERT: ERT discontinued D -14

Significant unmet need in Gaucher Type 1 Standard of Care – ERT Despite ERT, patients experience significant life-limiting disease burden including musculoskeletal pain and fatigue Registry data suggest disease progression despite ERT Incomplete Therapeutic Response is Common A clinically significant percentage of patients continue to exhibit bone pain, organomegaly and cytopenia after 10 years of ERT ~60% of patients fail to achieve at least 1 of 6 therapeutic goals after 4+ years of ERT ~25% of patients continue to suffer from physical limitations due to bone disease after 2 years of treatment Sources: Weinreb N et al, Amer J Hematol, 2008; Weinreb N et al, J Inherit Metab Dis, 2013; Giraldo P et al, Qual Life Res, 2005 Disease Manifestations Persist After 10 Years of ERT Persistence of: Non-splenectomized Patients Splenectomized Patients Anemia 12.4% 8.8% Thrombocytopenia 20.9%** 0.7%** Splenomegaly 37.4%** NA Hepatomegaly 14.3%** 18.8%** Bone Pain 42.9% 62.5% Bone Crisis 7.4% 16.7% *Following 10 years of treatment ~26% of patients were receiving between 45-150 U/kg EOW (96% of these individuals were receiving doses between 45-90 U/kg EOW) ** Higher persistence rates were observed when more severe manifestations were present at baseline Note: Total of 757 patients in registry as of this study; source: Weinreb N et al, J Inherit Metab Dis, 2013

Investigator-sponsored* Phase 1/2 study in Cystinosis OBJECTIVES PATIENTS ASSESS Safety Efficacy 6 patients adults and potentially adolescents 14–17 years old Using oral and ophthalmic cysteamine Cystine levels in granulocytes Vector Copy Number (VCN) Chimerism Renal, respiratory and endocrine function, ophthalmologic findings, muscle strength, growth, bone density, neurologic and psychometric measures Safety A Phase 1/2 study to determine the safety and efficacy of transplantation with autologous human CD34+ Hematopoietic Stem Cells (HSC) from Mobilized Peripheral Blood Stem Cells (PBSC) of patients with Cystinosis modified by ex vivo transduction using the pCCL-CTNS lentiviral vector Weeks 6-14 Months 6, 12, 18 & 24 Safety & efficacy assessments Day -140 Discontinue cysteamine eye drops Day -70 Mobilize stem cells Day -20 Discontinue oral cysteamine Day -5 Conditioning Day 0 Infuse AVR-RD-04 Day 28 Post-treatment assessment CYSTINOSIS PATIENTS * Sponsored by UCSD

Pompe preclinical program advancing Integrated 3-part approach GILT: Glycosylation-Independent Lysosomal Targeting Sources: Burton B et al, J Pediatr, 2017; Ausems M et al, Eur J Hum Genet, 1999; Gungor D et al, Orphanet J Rare Dis, 2011; Maga JA et al, J of Bio Chem, 2013 THE CHALLENGE Pompe requires 20x more ERT than Fabry or Gaucher Requires GAA activity restored to muscle and CNS AVROBIO’s APPROACH Potent transgene promoter GILT uptake tag plato™ for CNS impact mg Glycogen / g wet tissue 2 4 6 8 10 12 Dose mg/kg 0 5 10 15 20 HEART GILT-rhGAA rhGAA mg Glycogen / g wet tissue 2 4 6 8 10 12 Dose mg/kg 0 5 10 15 20 DIAPHRAGM GILT-rhGAA rhGAA GILT-tagged Recombinant Human (rh)GAA impacts levels of stored glycogen compared to non GILT-tagged Recombinant Human (rh)GAA in a Pompe mouse model

plato™ –– AVROBIO’s foundation for worldwide commercialization Beginning-to-end manufacturing platform Redefines manufacturing best practices Optimized for performance

2016 2017 2018 2019 Multiple plato™ IND and CTA regulatory clearances achieved 1H 2019 Cryo-preservation 3-day cycle time Mobilized, GCSF, Plerixafor Algorithm finalized CBER Pre-IND Conditioning Ad Board GMP ready USA/CAN Cryo-preservation 3-day cycle time Mobilized, GCSF, Plerixafor Algorithm finalized CBER Pre-IND GMP ready USA/CAN Initiate EU transfer Conditioning Ad Board Vector design Dev LV prod platform Development for automated process Tech transfer to US CMO FABRY GAUCHER Vector design Development for automated process Dev LV Prod Platform Tech transfer to US CMO plato™ CLEARED In-vitro comparability plato™ CLEARED Tech transfer to AUS Tech transfer to AUS plato cleared: CA, February plato™ CLEARED plato™ CLEARED Note: plato in Fabry cleared for use in US via IND, in Canada via protocol and CMC CTA amendment, and in AUS via CTN and HREC clearance; plato in Gaucher cleared for use in Canada via CTA and protocol CTA amendment

TDM Academic platform plato 0 2 1 plato™ optimized for performance Therapeutic Drug Monitored (TDM) Conditioning Proprietary Vector Toolbox VCN Transduction Efficiency Enzyme Activity 1 2 3 4 DAY TOXICITY ENGRAFTMENT Distribution BRAIN BONE MUSCLE Academic platform plato KIDNEY PROMOTERS OPTIMIZED TRANSCRIPTION TAGS CODON OPTIMIZATION KOZAK SEQUENCE OPTIMIZED VECTOR 0 15 10 5 Academic platform plato Fold AGA Activity Increase Vector Copy Number % Transduction Note: Data from appropriate runs from normal donors and patients are included in the analysis; Data cutoff March 12, 2019 OPTIMIZED TRANSLATION HEART 80% 60% 40% 20% 0% 100% 3 20

DRUG PRODUCT plato™ platform designed to be scalable for commercial supply VECTOR (200 L scale bioreactor runs (109 titre)) 4 production suites ~12 runs per year per suite ~50 patients per run 2,400 PATIENTS ANNUALLY 3 global production suites 8 automated units per suite 100 patients per unit per year 2,400 PATIENTS ANNUALLY


Multiple near-term milestones anticipated FABRY Continued recruitment in FAB-201, with dosing of first Fabry patient under platoTM in 2019 FAB-201 clinical sites to expand into USA in 2019 GAUCHER Enroll first patient in GAU-201 in Q1 2020 with dosing in Q2 2020 Dose first patient in investigator-sponsored trial in 2019 Pre-clinical IND-enabling study to be initiated in 2019 CYSTINOSIS POMPE

Appendix

Precedent for use of kidney biopsy data for FDA approval of drug candidate for Fabry disease Classic Fabry disease (AGA activity <1%) NOTE: For informational purposes; differences exist between trial designs and subject populations; AVROBIO has not conducted any head-to-head trials comparing migalastat to AVR-RD-01 Classic Fabry patient level data 0-6 months randomized clinical trial and 6-12 months open label extension 28% average reduction (at 6 months from baseline) 46% average reduction (average of patients with 12 month data) 7/9 males ≥ 50% reduction (at 6 months from baseline) 45 Amenable patients* (16 males / 29 females) Migalastat approved on % reduction in GL-3 inclusions per KIC as compared to placebo Group Migalastat (BL –M6) Placebo (BL –M6) Males (N=16) 5/7 (71%) -1.10 (-1.94, -0.02) 4/9 (44%) -0.03 (-1.00, 1.69) Patients with baseline GL-3 ≥ 0.3 (N=17; 9 males, 8 females) 7/9 (78%) -0.91 (-1.94, 0.19) 2/8 (25%) -0.02 (-1.00, 1.69) Patients with baseline GL-3 < 0.3 (N=28; 7 males, 21 females) 6/16 (38%) -0.02 (-0.10, 0.26) 7/12 (58%) -0.05 (-0.16, 0.14) Treatment Group n Baseline Median (min, max) Month 6 Median (min, max) Change from Baseline Median (min, max) Average number of GL-3 inclusions per KIC (N=13) Galafold 7 3.6 (0.2, 6.0) 2.6 (0.1, 6.0) -0.7 (-1.7, 1.2) Placebo 6 1.8 (0.1, 2.8) 2.0 (0.05, 4.3) -0.04 (-0.5, 1.5) Male Patients with the Classic Phenotype Migalastat (Months 0-24) Placebo (Months 0-6) → Migalastat (Months 6-24) #1 #2 #3 #4 #5 #6 #7 #8 #9 #10 #11 #12 #13 #14 PTC GL-3 inclusions at BL 0.16 0.03 n/a 5.69 1.22 n/a 2.88 2.41 1.55 0.16 0.03 0.11 0.94 0.88 Change in PTC GL-3 inclusions from BL to M6 -0.08 0.01 n/a -1.77 -1.10 n/a -1.25 1.21 -0.21 0.01 0.09 -0.07 1.94 -0.83 Change in PTC GL-3 inclusions from BL/M6b to M12 -0.12 n/a n/a -1.92 n/a n/a -0.81 -0.94 -1.13 -0.09 -0.05 n/a -2.28 0.06 Source: Germain D et al, Genetics in Medicine, 2019

Hematopoietic reconstitution occurs in two distinct phases A few thousand long-term engrafting cells stably sustain levels of transgene product First wave of short-term progenitor cells start to exhaust with progressive takeover by a smaller population of long-term engrafting cells Source: Biasco L et al, Cell Stem Cell, 2016 Clonal Population Time after GT (months) 6 - 12 24 - 48 0 - 3 Long-term engrafting cells Short-term progenitor cells
